 FHIR IG analytics
FHIR IG analytics| Package | tw.gov.mohw.nhi.pas |
| Resource Type | StructureDefinition |
| Id | ResponseModel |
| FHIR Version | R4 |
| Source | https://nhicore.nhi.gov.tw/pas/https://build.fhir.org/ig/TWNHIFHIR/pas/StructureDefinition-ResponseModel.html |
| URL | https://nhicore.nhi.gov.tw/pas/StructureDefinition/ResponseModel |
| Version | 1.0.7 |
| Status | active |
| Date | 2025-08-11T10:05:15+00:00 |
| Name | ResponseModel |
| Title | 回覆(Response)癌症用藥癌藥事前審查之資料模型 |
| Realm | tw |
| Authority | national |
| Description | 回覆(Response)癌症用藥癌藥事前審查之資料模型,此邏輯模型為定義癌症用藥事前審查情境下使用的所有資料欄位。 為了便於實作者快速理解,資料欄位會使用易於理解的命名,實作者再透過邏輯模型中的功能頁籤「Mappings」瞭解各資料欄位實際使用本IG的哪個Profiles的哪個資料項目(element)。亦可配合[視覺化邏輯模型圖](vision.html#bundle%E6%9E%B6%E6%A7%8B%E5%9C%96)進行欄位對應 |
| Type | https://nhicore.nhi.gov.tw/pas/StructureDefinition/ResponseModel |
| Kind | logical |
No resources found
No resources found
Note: links and images are rebased to the (stated) source
Generated Narrative: StructureDefinition ResponseModel
| Name | Flags | Card. | Type | Description & Constraints |
|---|---|---|---|---|
 | 0..* | Base | 回覆(Response)癌症用藥癌藥事前審查之資料模型 | |
 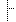 | 1..1 | dateTime | 核定日期 | |
 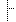 | 1..1 | string | 案件受理狀態 | |
 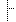 | 0..1 | CodeableConcept | 受理審查案件核定註記 | |
 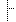 | 0..1 | decimal | 核定數量 | |
  | 0..1 | CodeableConcept | 個別醫令核定註記 | |
{
"resourceType": "StructureDefinition",
"id": "ResponseModel",
"text": {
"status": "extensions",
"div": "<!-- snip (see above) -->"
},
"url": "https://nhicore.nhi.gov.tw/pas/StructureDefinition/ResponseModel",
"version": "1.0.7",
"name": "ResponseModel",
"title": "回覆(Response)癌症用藥癌藥事前審查之資料模型",
"status": "active",
"date": "2025-08-11T10:05:15+00:00",
"publisher": "衛生福利部中央健康保險署",
"contact": [
{
"name": "衛生福利部中央健康保險署",
"telecom": [
{
"system": "url",
"value": "https://www.nhi.gov.tw"
}
]
}
],
"description": "回覆(Response)癌症用藥癌藥事前審查之資料模型,此邏輯模型為定義癌症用藥事前審查情境下使用的所有資料欄位。 \n為了便於實作者快速理解,資料欄位會使用易於理解的命名,實作者再透過邏輯模型中的功能頁籤「Mappings」瞭解各資料欄位實際使用本IG的哪個Profiles的哪個資料項目(element)。亦可配合[視覺化邏輯模型圖](vision.html#bundle%E6%9E%B6%E6%A7%8B%E5%9C%96)進行欄位對應",
"fhirVersion": "4.0.1",
"kind": "logical",
"abstract": false,
"type": "https://nhicore.nhi.gov.tw/pas/StructureDefinition/ResponseModel",
"baseDefinition": "http://hl7.org/fhir/StructureDefinition/Base",
"derivation": "specialization",
"snapshot": {
"extension": [
{
"url": "http://hl7.org/fhir/tools/StructureDefinition/snapshot-base-version",
"valueString": "4.0.1"
}
],
"element": [
{
"id": "ResponseModel",
"path": "ResponseModel",
"short": "回覆(Response)癌症用藥癌藥事前審查之資料模型",
"definition": "回覆(Response)癌症用藥癌藥事前審查之資料模型,此邏輯模型為定義癌症用藥事前審查情境下使用的所有資料欄位。 \n為了便於實作者快速理解,資料欄位會使用易於理解的命名,實作者再透過邏輯模型中的功能頁籤「Mappings」瞭解各資料欄位實際使用本IG的哪個Profiles的哪個資料項目(element)。亦可配合[視覺化邏輯模型圖](vision.html#bundle%E6%9E%B6%E6%A7%8B%E5%9C%96)進行欄位對應",
"min": 0,
"max": "*",
"base": {
"path": "Base",
"min": 0,
"max": "*"
},
"isModifier": false
},
{
"id": "ResponseModel.approveDate",
"path": "ResponseModel.approveDate",
"short": "核定日期",
"definition": "核定日期",
"min": 1,
"max": "1",
"base": {
"path": "ResponseModel.approveDate",
"min": 1,
"max": "1"
},
"type": [
{
"code": "dateTime"
}
]
},
{
"id": "ResponseModel.acptNo",
"path": "ResponseModel.acptNo",
"short": "案件受理狀態",
"definition": "案件受理狀態",
"min": 1,
"max": "1",
"base": {
"path": "ResponseModel.acptNo",
"min": 1,
"max": "1"
},
"type": [
{
"code": "string"
}
]
},
{
"id": "ResponseModel.approveClaimComment",
"path": "ResponseModel.approveClaimComment",
"short": "受理審查案件核定註記",
"definition": "受理審查案件核定註記。1:同意 | 2:不予同意 | 3:部份同意 | 4:補件 | 5:退件",
"min": 0,
"max": "1",
"base": {
"path": "ResponseModel.approveClaimComment",
"min": 0,
"max": "1"
},
"type": [
{
"code": "CodeableConcept"
}
]
},
{
"id": "ResponseModel.approveNum",
"path": "ResponseModel.approveNum",
"short": "核定數量",
"definition": "核定數量",
"min": 0,
"max": "1",
"base": {
"path": "ResponseModel.approveNum",
"min": 0,
"max": "1"
},
"type": [
{
"code": "decimal"
}
]
},
{
"id": "ResponseModel.approveItemComment",
"path": "ResponseModel.approveItemComment",
"short": "個別醫令核定註記",
"definition": "個別醫令核定註記。0:審核中 | 1:同意 | 2:不予同意 | 3:部份同意 | 4:補件 | 5:退件 | 6:不予同意:對應手術亦不支付 | 7:改核:如審查核定意見",
"min": 0,
"max": "1",
"base": {
"path": "ResponseModel.approveItemComment",
"min": 0,
"max": "1"
},
"type": [
{
"code": "CodeableConcept"
}
]
}
]
},
"differential": {
"element": [
{
"id": "ResponseModel",
"path": "ResponseModel",
"short": "回覆(Response)癌症用藥癌藥事前審查之資料模型",
"definition": "回覆(Response)癌症用藥癌藥事前審查之資料模型,此邏輯模型為定義癌症用藥事前審查情境下使用的所有資料欄位。 \n為了便於實作者快速理解,資料欄位會使用易於理解的命名,實作者再透過邏輯模型中的功能頁籤「Mappings」瞭解各資料欄位實際使用本IG的哪個Profiles的哪個資料項目(element)。亦可配合[視覺化邏輯模型圖](vision.html#bundle%E6%9E%B6%E6%A7%8B%E5%9C%96)進行欄位對應"
},
{
"id": "ResponseModel.approveDate",
"path": "ResponseModel.approveDate",
"short": "核定日期",
"definition": "核定日期",
"min": 1,
"max": "1",
"type": [
{
"code": "dateTime"
}
]
},
{
"id": "ResponseModel.acptNo",
"path": "ResponseModel.acptNo",
"short": "案件受理狀態",
"definition": "案件受理狀態",
"min": 1,
"max": "1",
"type": [
{
"code": "string"
}
]
},
{
"id": "ResponseModel.approveClaimComment",
"path": "ResponseModel.approveClaimComment",
"short": "受理審查案件核定註記",
"definition": "受理審查案件核定註記。1:同意 | 2:不予同意 | 3:部份同意 | 4:補件 | 5:退件",
"min": 0,
"max": "1",
"type": [
{
"code": "CodeableConcept"
}
]
},
{
"id": "ResponseModel.approveNum",
"path": "ResponseModel.approveNum",
"short": "核定數量",
"definition": "核定數量",
"min": 0,
"max": "1",
"type": [
{
"code": "decimal"
}
]
},
{
"id": "ResponseModel.approveItemComment",
"path": "ResponseModel.approveItemComment",
"short": "個別醫令核定註記",
"definition": "個別醫令核定註記。0:審核中 | 1:同意 | 2:不予同意 | 3:部份同意 | 4:補件 | 5:退件 | 6:不予同意:對應手術亦不支付 | 7:改核:如審查核定意見",
"min": 0,
"max": "1",
"type": [
{
"code": "CodeableConcept"
}
]
}
]
}
}